 UMIN Clinical Trial Registry System (UMIN-CTR) Operation Manual
UMIN Clinical Trial Registry System (UMIN-CTR) Operation Manual
1.About access
A UMIN ID and password are required to register clinical trials with UMIN-CTR and update registered trial information.
・How to get UMIN ID for clinical trial registration
・Changing your password (when you do not know your current one) Resetting your password
2.Checklist
2-1)Minimum items required for registration
- UMIN-CTR defines the minimum required items (essential items) for registration of clinical trials, and it is a specification that formal registration (set) cannot be performed unless all the required items are filled.
- FAQ
2-2)training
- For practice of operation and registration. Test system - Practice registering and updating trial information
- A UMIN ID is required.
- It's for practice. Official registration is not possible through this system. Please be careful.
2-3)cost
There is no cost for clinical trial enrollment in UMIN-CTR.
3.Register as new
3-1)Registration of basic information form
Please log in with your UMIN ID and password from [Entering your trial information.] on the UMIN Clinical Trial Registration System (UMIN-CTR) website.
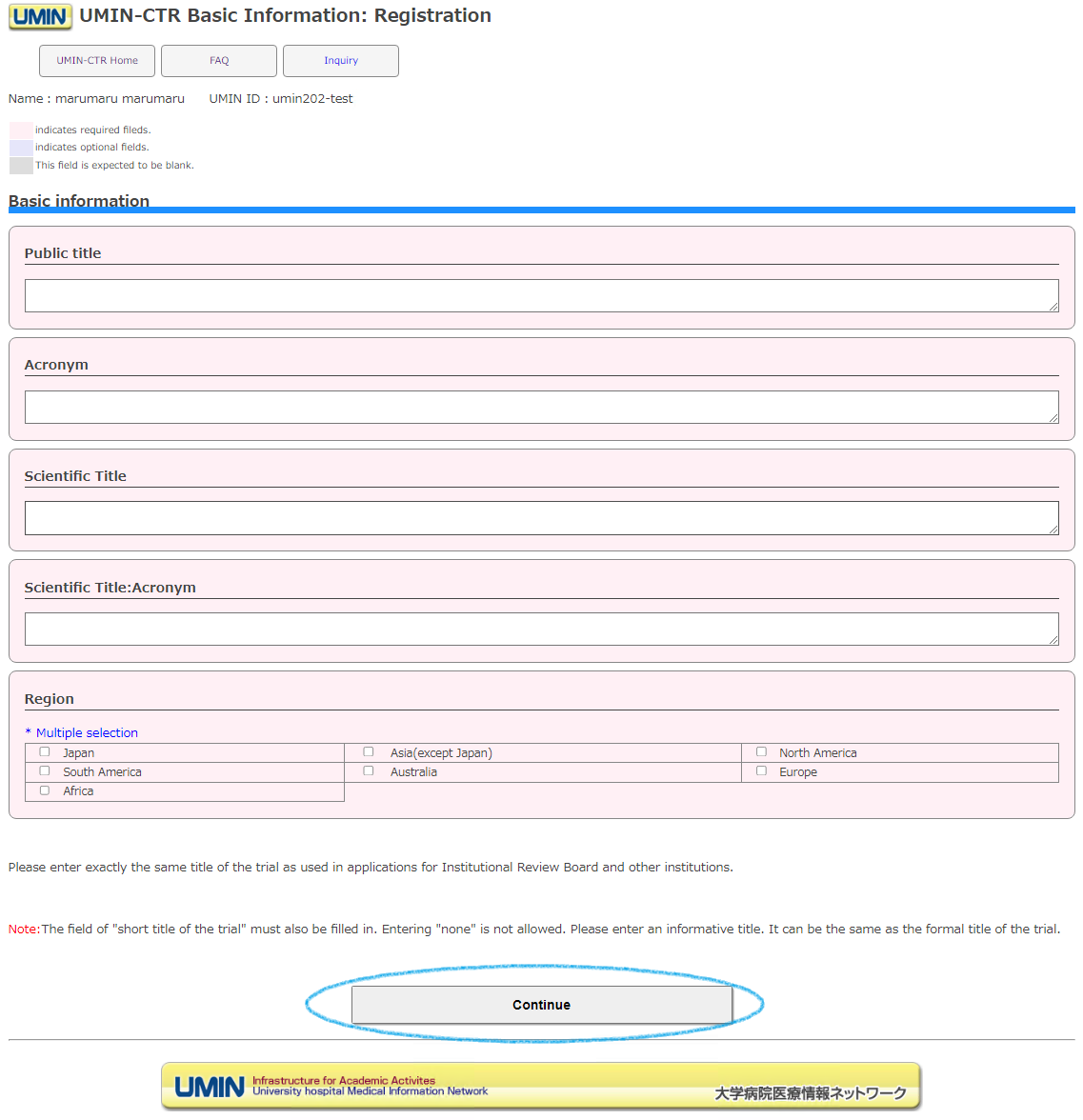
After logging in, a basic information form will be displayed. Enter the public title, etc., and press "Continue".
・If the name of the scientific study has been notified to an ethics committee or other institution, please be sure to enter the same name.
・When registering multiple tests with the same test name, we ask that you add A, B, 1, 2, etc. to the "abbreviation" so that they can be distinguished. The scientific test name "abbreviation" will be displayed on the screen open to the public, so please be considerate.
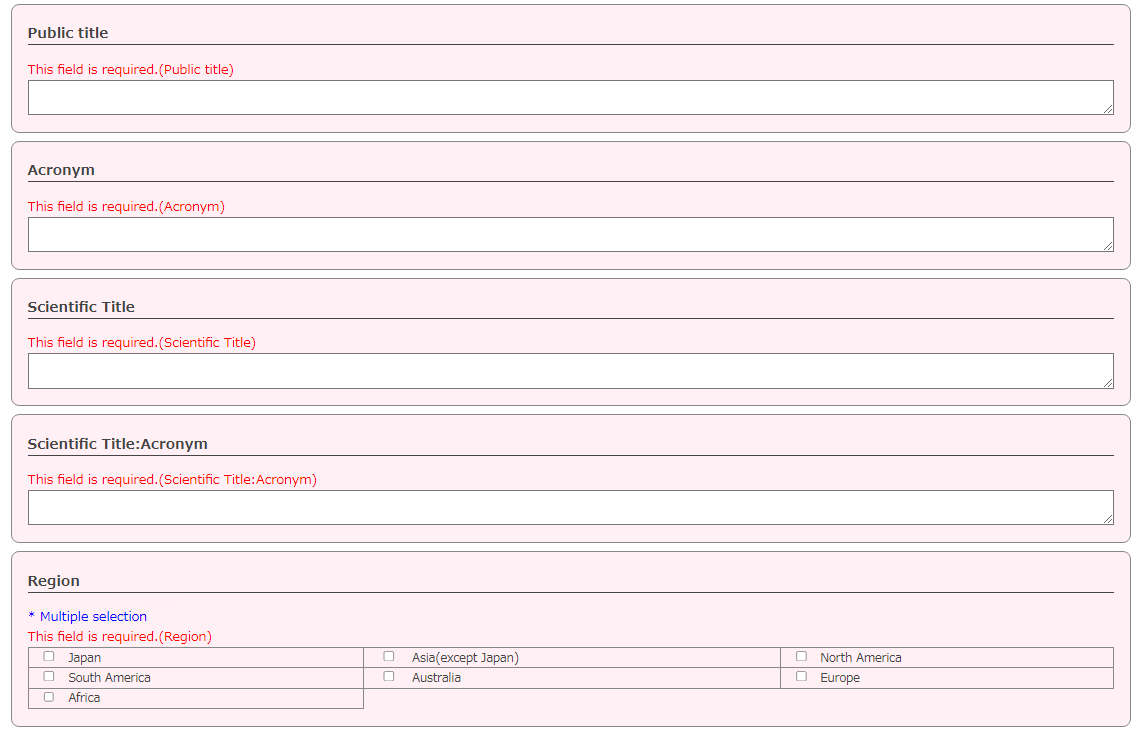
If you do not enter a required item or the input value is not correct, an error will be output in red, so please correct it.
Items with a pink background are required items, but depending on the input value, required items and optional items may change.
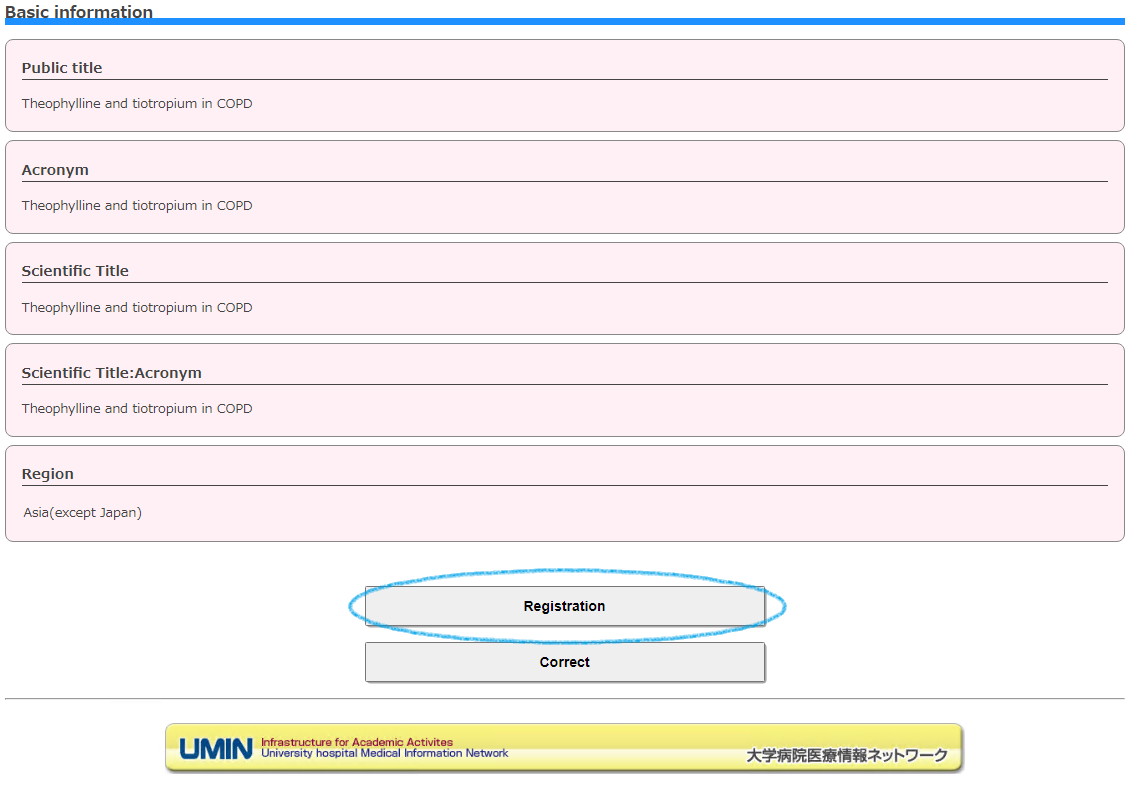
If there is no problem with the input value, the "Registration" button will be output.
Please proceed from "Registration" to "Continue".
3-2)Registration of each form
At this point, it is not an official registration, but the reception has been completed. A receipt number (R000000000) will be issued on the next screen.

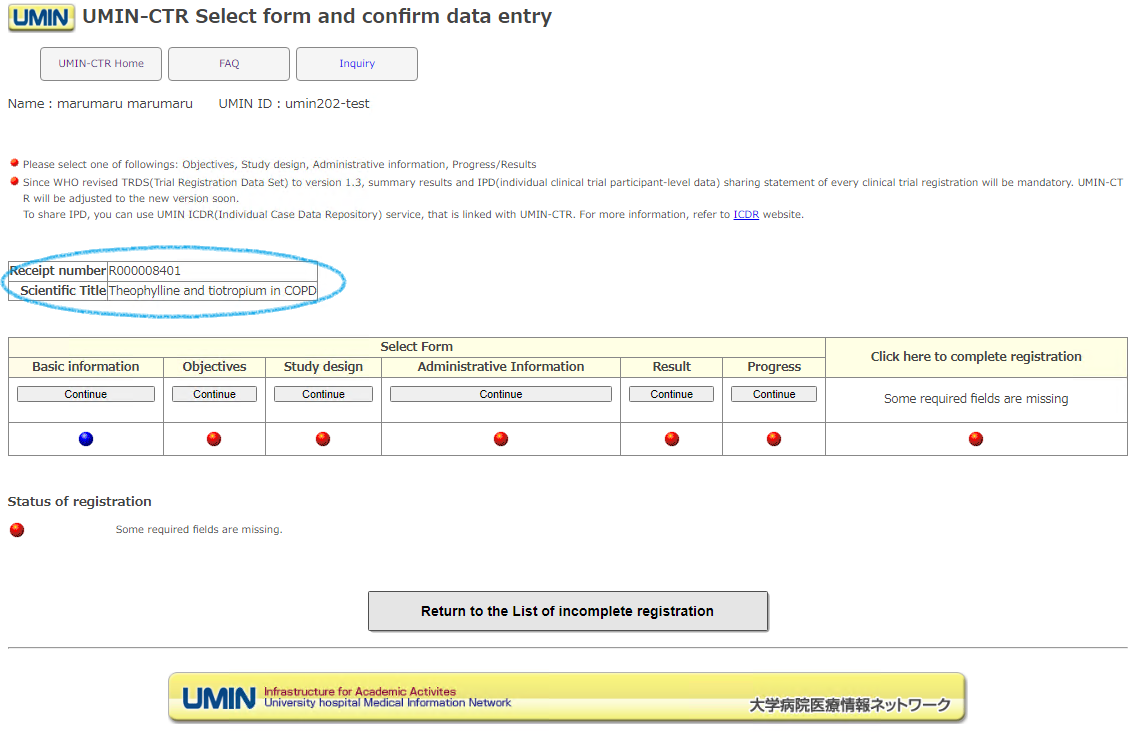
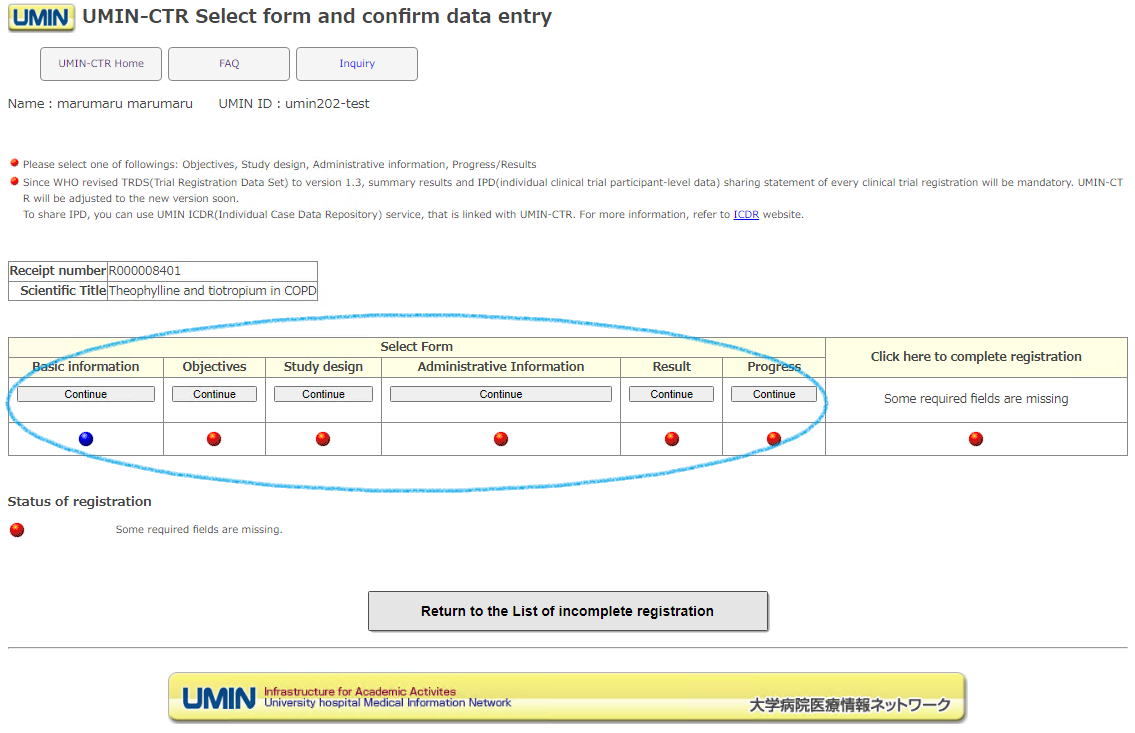
In order to formally register (set), it is necessary to fill in all forms.
Please access each form from the [Continue] button.
When registering information, it is checked whether the required items and data types are correct.
If there is a problem, the [Registration] button will not appear, so correct the error message and register.
In addition, if you move to another screen without confirming with the [Registration] button while filling in each form, the input contents will not be retained. Please be careful.
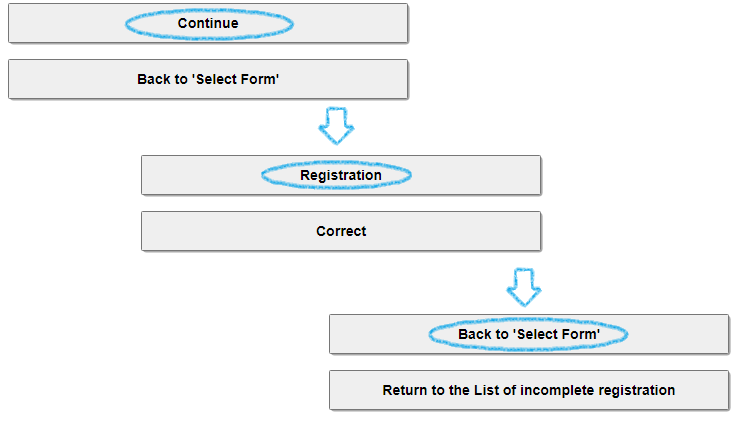
After completing the form input, please repeat the registration for each form in the order of [Continue] ⇒ [Registration] ⇒ [Back to 'Select form'] at the bottom of the screen.
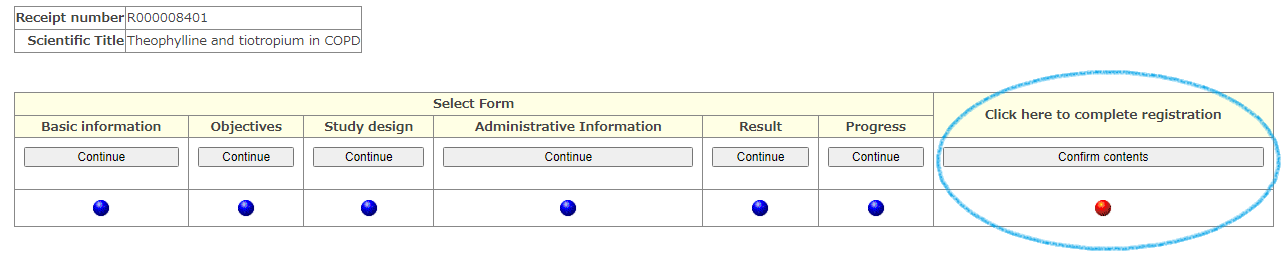
When all forms have been entered, the [Confirm input] button will be displayed in the final confirmation column of the data. Proceed to the next screen and check the registration details.
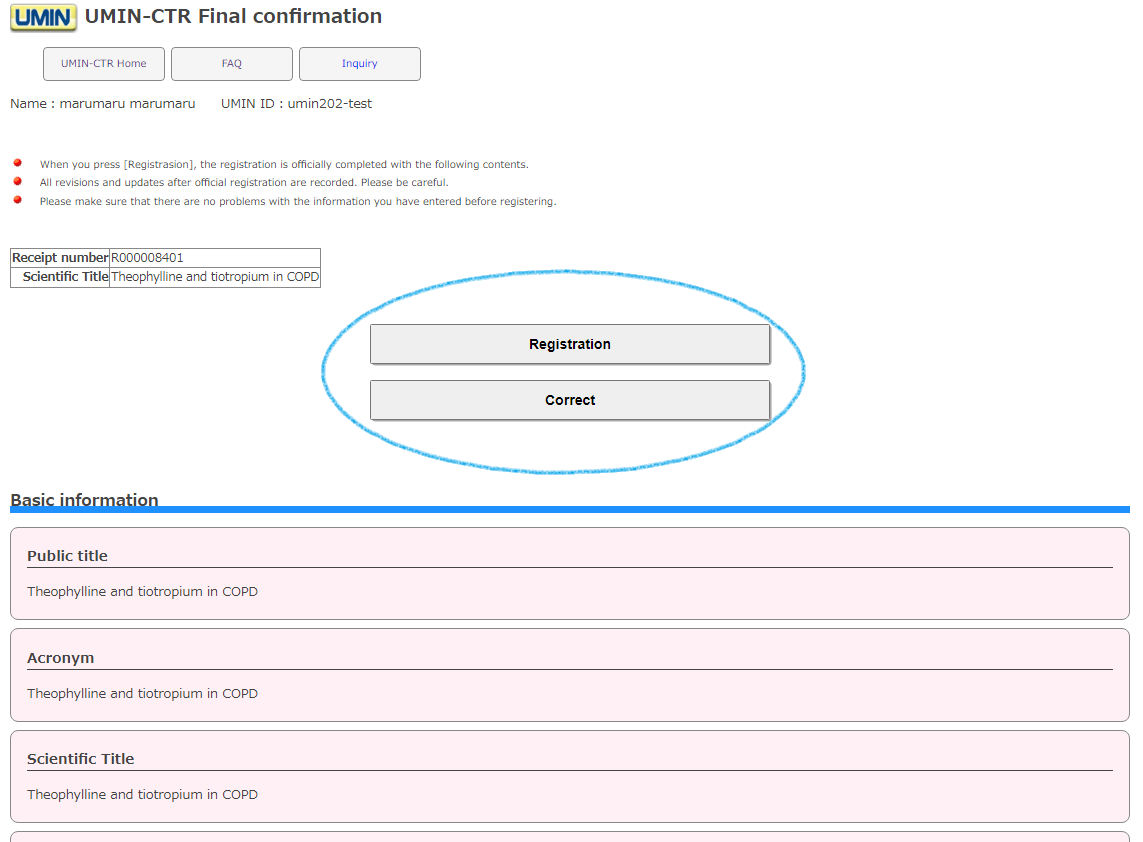
The entered information will be displayed
[Registration]
Please note that the registration (set) will be formally made and you will not be able to cancel it later. In addition, since the history of corrections and updates after formal registration (set) is recorded, please check it thoroughly before executing it.
[Correct]
You will return to the previous screen (form selection screen), so if you need to make any corrections, please click here.
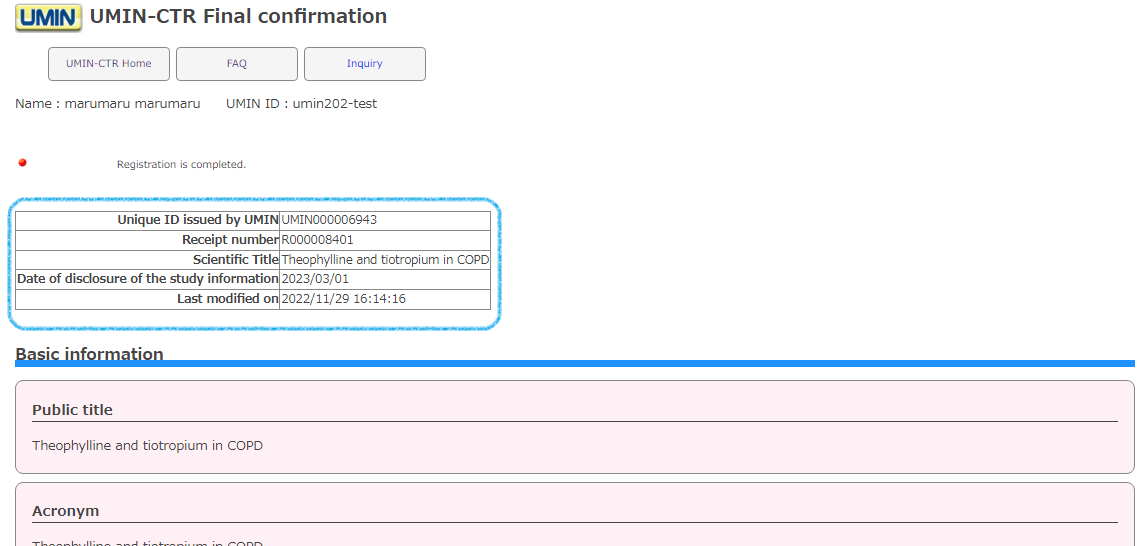
You have officially registered (set) with [registration].
A UMIN exam ID (UMIN000000000) has been issued, so please make a note of it.
*At this point, you will no longer be able to cancel your registration exam.
Note! !
[Date of disclosure of the study information] should be the date on which the registration test information is announced.The date of publication in the clinical trial registration system will be treated as the official registration date of the clinical trial.
This item is also sent to WHO (ICTRP), and [Date of disclosure of the study information] is treated as [Date of registration] and [Anticipated trial start date] is treated as [Date of first enrollment].
4.Update/correction of registered information
4-1)Updates, corrections, and withdrawals before official registration
If the work is interrupted while the registration (set) has not been officially completed (Unique ID issued by UMIN00000000 has not been issued), please access from the [Incomplete registration] button on the UMIN-CTR HP. . Until the official registration is completed, it will be accessed from here.
*UMIN ID and password are required for access.
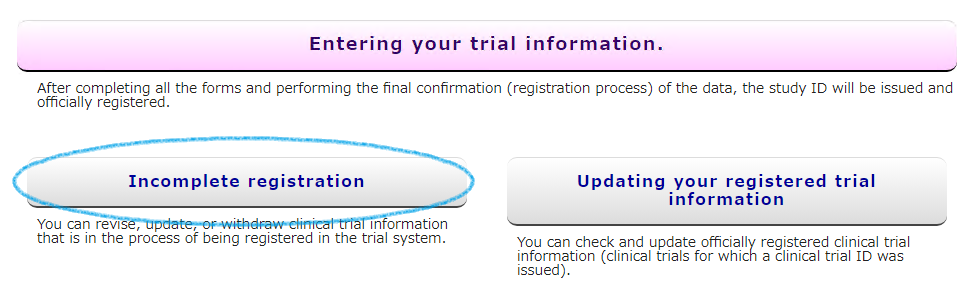
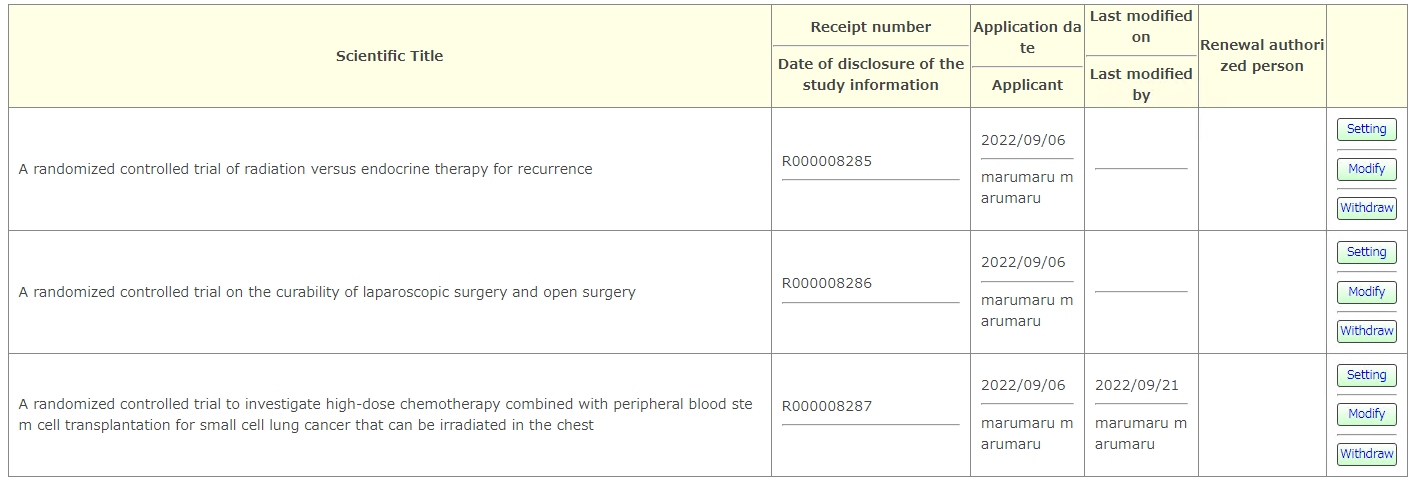
Please access with UMIN ID and password. Tests before official registration will be displayed with the accessed UMIN ID.
[setting] You can set so that anyone other than the registrant can update the test information.
[Modify] You can update the test information in the middle of registration and perform official registration.
[Withdraw]You can withdraw a test before official registration.
Check "3-2) Registration of each form" for the input method.
Information on officially registered (set) clinical trials will be published on the date entered in [Date of disclosure of the study information] and will be searchable. Please set the date to publish the exam information.
4-2)Update after formal registration
If you want to update your exam information after you have officially registered (Unique ID UMIN00000000 has been issued), please access from the [Updating your registered trial information] button on the UMIN-CTR HP.
*UMIN ID and password are required for access.
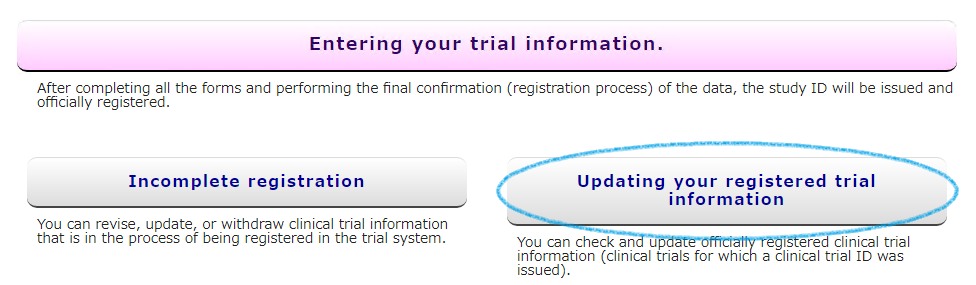
If there are any changes in the registered clinical trial information (changes in the protocol, study progress, etc.), please reflect the changes in UMIN-CTR as soon as possible.
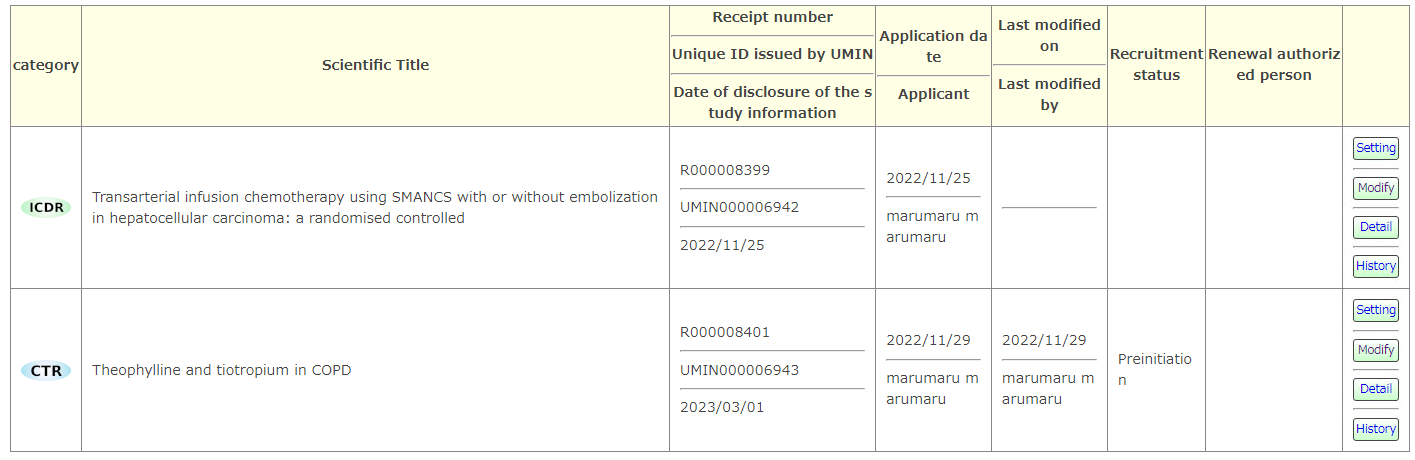
Exams that have been officially registered with the accessed UMIN ID and exams that have been set as an authorized updater will be displayed.
[setting] You can set so that anyone other than the registrant can update the test information.
[Modify] Registered information can be updated and corrected.
- Click "Modify" to the right of the applicable clinical trial
- Click "Continue" on the appropriate form
- After completing the correction, click [Continue] at the bottom of the screen.
- If there is no problem with the contents, click [Registration] to complete the work
- If you want to modify another form, start from [2]
[History] The update history is recorded and can be checked. General readers can also display the history of only the public information of the searched exam.
If you proceed from [Modify], you will be linked to each form, so please update to the latest information when there is progress.
Also, in officially registered clinical trials, UMIN-ICDS can be used as an additional function of UMIN-CTR.
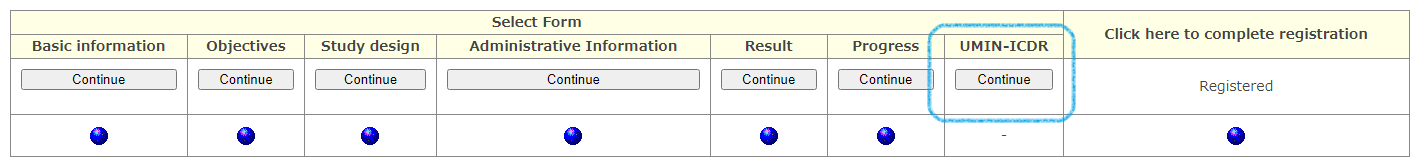
For clinical trials that are open to the public, a progress confirmation email will be automatically sent to the information sending organization and IRB every 6 months from the date of official registration. If there is an update, etc., please check the content and respond accordingly. Progress confirmation e-mails will be sent until the actual data trial progress status becomes "Completed" or "Terminated".
Information on officially registered (set) clinical trials will be published after the date entered in [Date of disclosure of the study information].
The search target will also be from [Date of disclosure of the study information]. Please be careful.
5.Viewing/confirming publicly available information
You can browse published clinical trials in the [List and search of clinical trials registered and published in the UMIN clinical trial registration system] section of the UMIN Clinical Trial Registration System (UMIN-CTR) website.
[List] A list of clinical trials published on UMIN-CTR will be displayed.
[Search] You can search published clinical trials by registered content.
[Free word search] Keyword search can be performed with AND or OR.
The search target will be from the date entered in [Date of disclosure of the study information] of the Administrative Information form.