 UMIN Individual Case Data Sharing System(UMIN-ICDS)
UMIN Individual Case Data Sharing System(UMIN-ICDS)
01.Storage of clinical trial data registered in UMIN-CTR
This manual explains the flow after the completion of UMIN-CTR official registration of CTR & ICDS (blue arrow).
If it is "ICDS only" and registration has already been completed, the same operation method will be used.
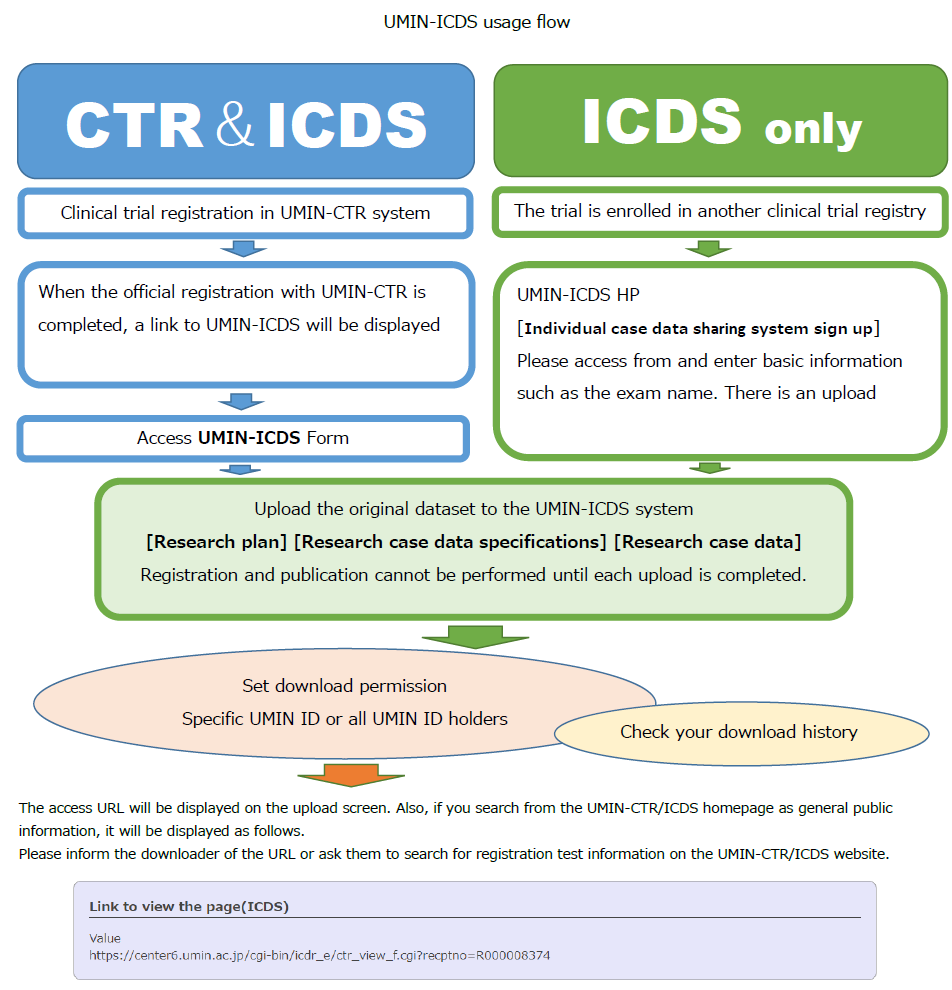
01-1)Transition to upload screen
You can store the data of studies that have been officially registered (UMIN study ID (UMIN000000000) has been issued) in the UMIN clinical trial registration system (UMIN-CTR).
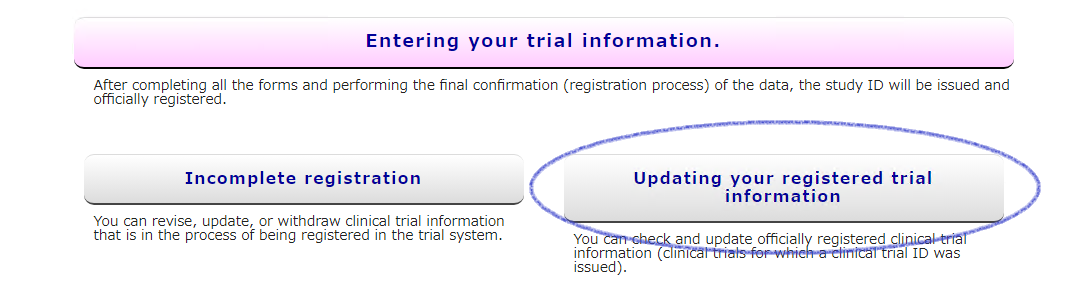
Access from [Updating your registered trial information] on the UMIN-CTR HP.
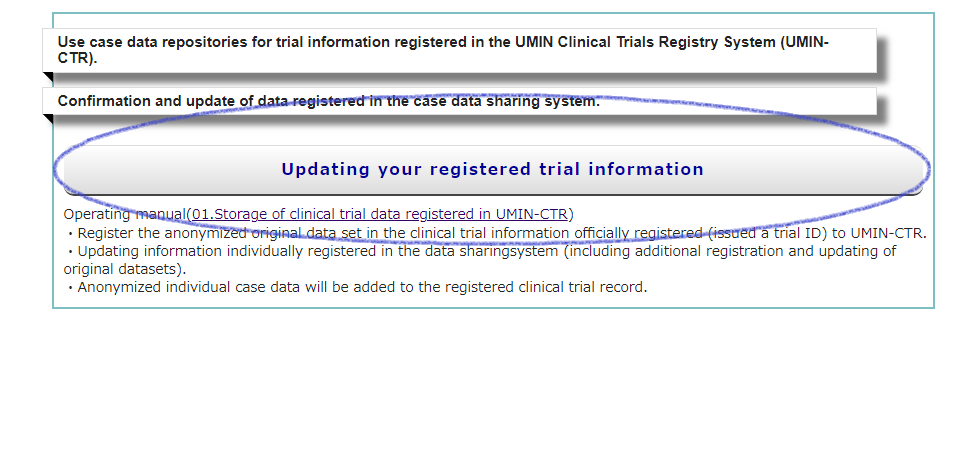
Or access from [Additional Registration/Update] on the UMIN-ICDS HP.
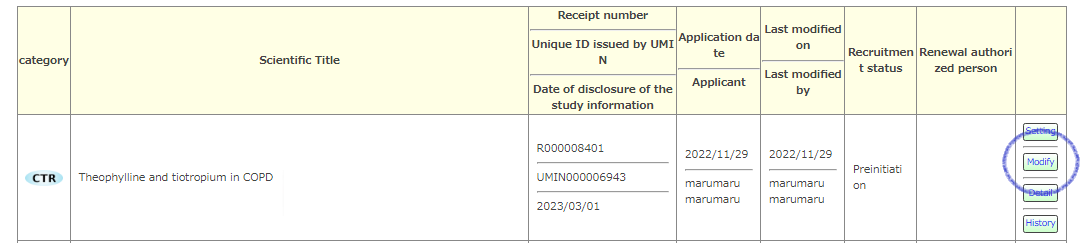
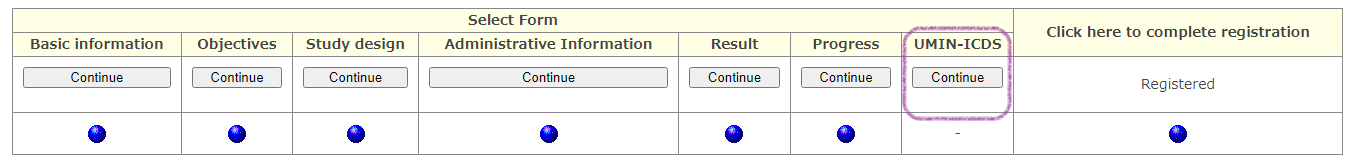
From [Modify] of the target exam, you will be transferred to the form selection screen.
A button dedicated to [UMIN-ICDS] will appear on the form selection screen for studies that have been officially registered with UMIN-CTR, so you can proceed to the ICDS form from here and save the data set.
*This screen does not appear if you have registered with "ICDS only". The information input screen is displayed directly and you can update the registration information and upload a new file.
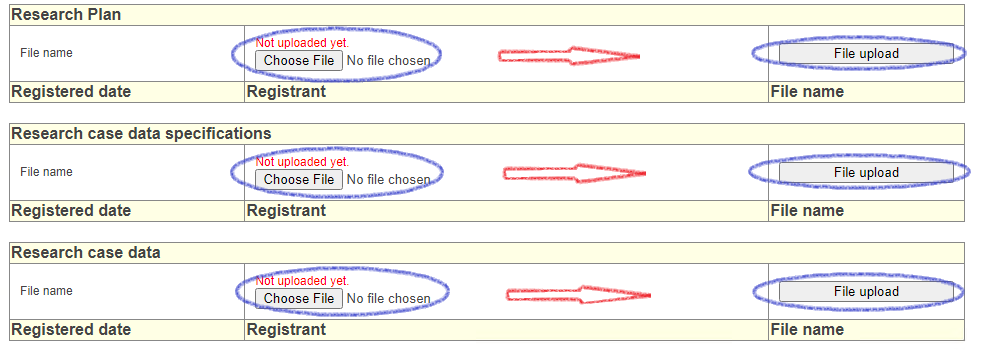
[Research Plan] [Research case data specifications] [Research case data] Please upload each data file. You cannot upload 3 files at the same time. Please upload one by one.
! Caution !
●Downloaders cannot access unless the "research plan", "research case data specifications", and "research case data" have been uploaded. All uploads are required.
●Since each file will be uploaded, if there are multiple files, please compress them into one.
●Personal information (including medical record numbers) cannot be posted. You must anonymize it. The method of anonymization is left to the researcher.
●Files can be uploaded any number of times. Authorized downloaders can only access the latest uploaded files.
●Once uploaded, it cannot be deleted. Please be careful when uploading.
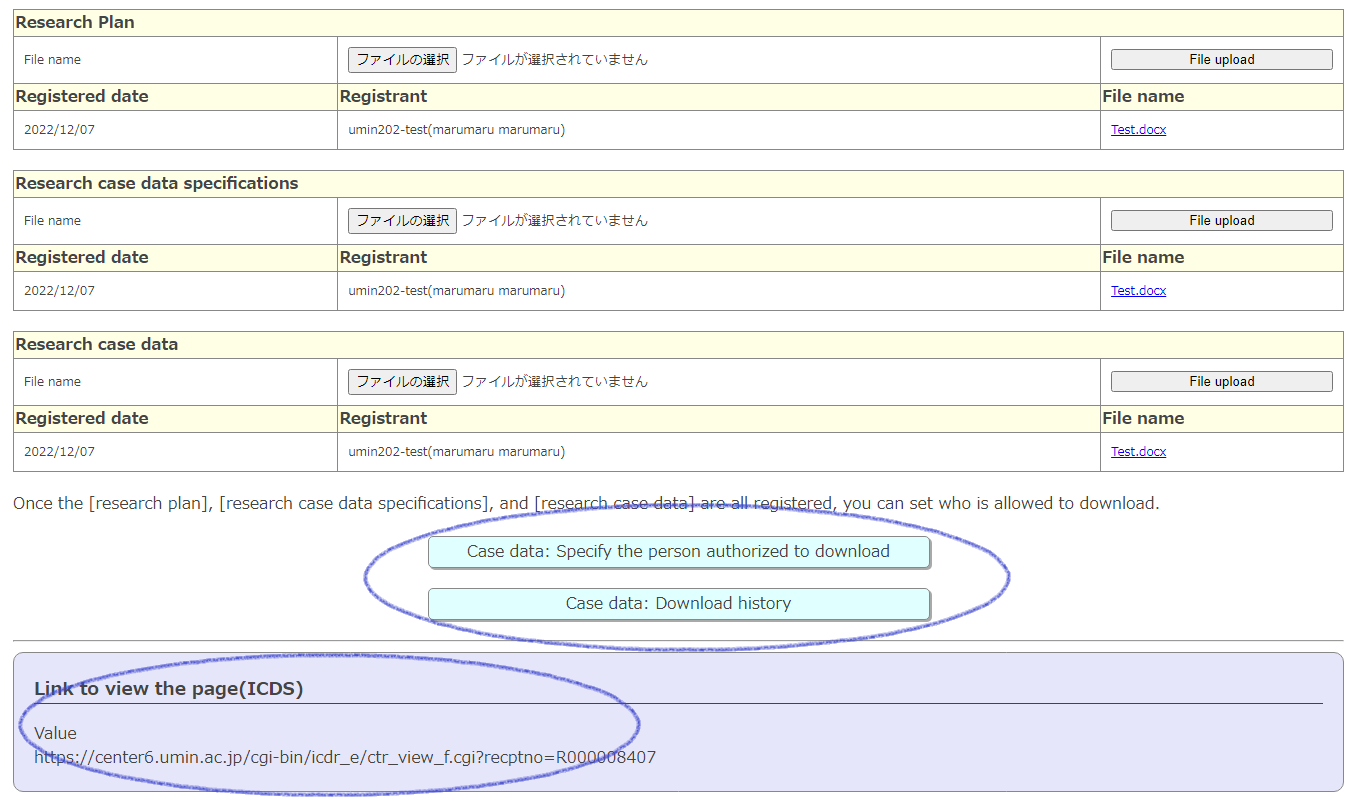
When the “research plan”, “research case data specifications”, and “research case data” are all complete, a light blue button will appear at the bottom so that you can set the download permission and check the history of the download.
Also, when you set a specific download permission person, please inform us of the URL at the bottom. It will be the URL of the link screen to the download file.
※Since the UMIN ID authorized by the registrant is used for authentication, only the authorized person can download the file.
01-2)When setting specific downloaders
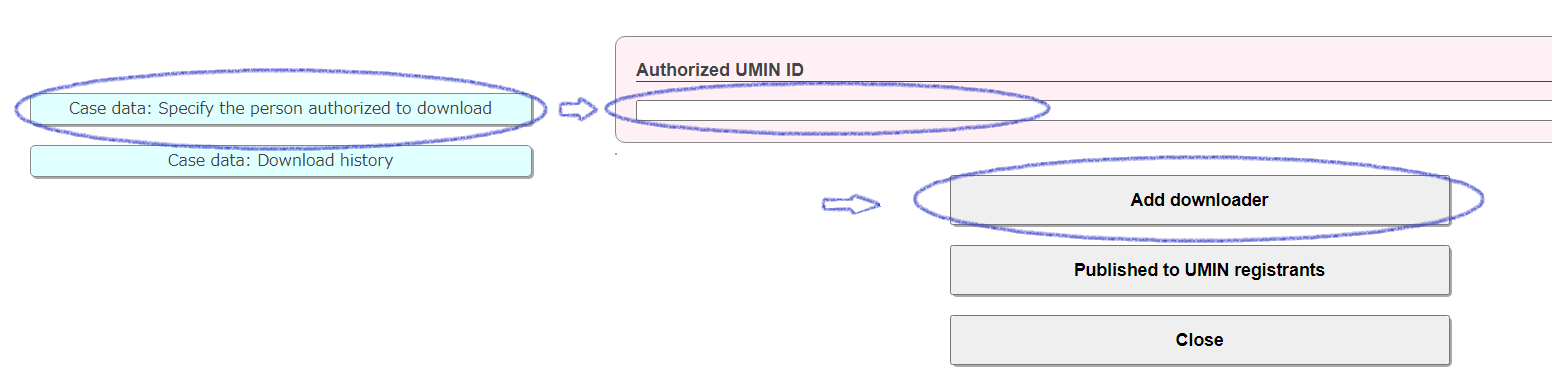
Case data: Specify the person authorized to download
Enter the UMIN ID of the person who is permitted to download in the "Authorized UMIN ID" field at the top of the transition form and click the [Add downloader] button. You can set up specific authorized people to share your data with. Cancellation (deletion) is possible.
You can register one ID at a time, but there is no upper limit.
01-3)When allowing all UMIN ID holders
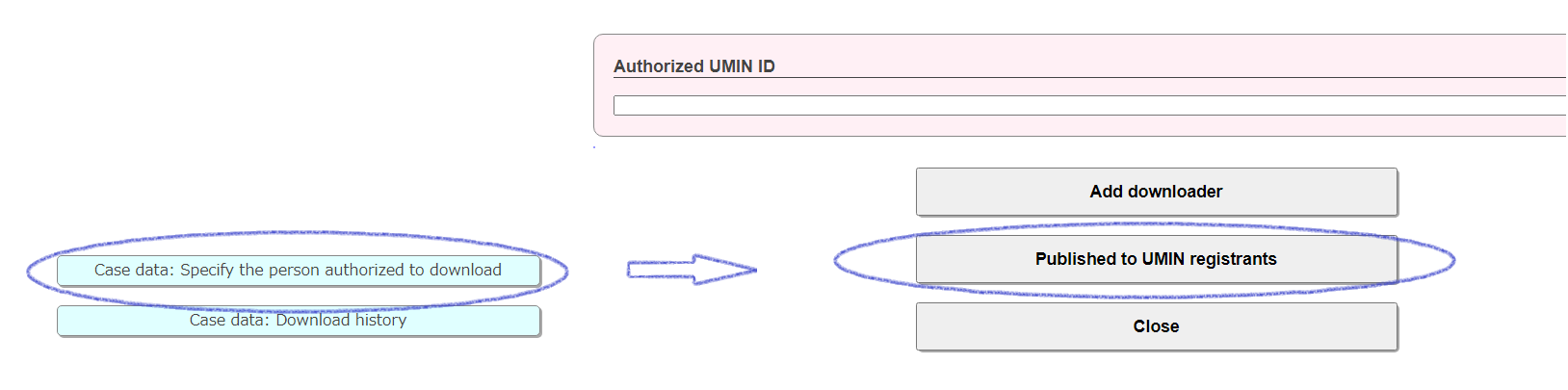
Case data: Specify the person authorized to download
By clicking "Published to UMIN registrants" on the transition form, access will be granted to all UMIN ID holders. All users who have a UMIN ID will be permitted, so please check carefully before setting. Cancellation (deletion) is possible.
01-4)Check download history
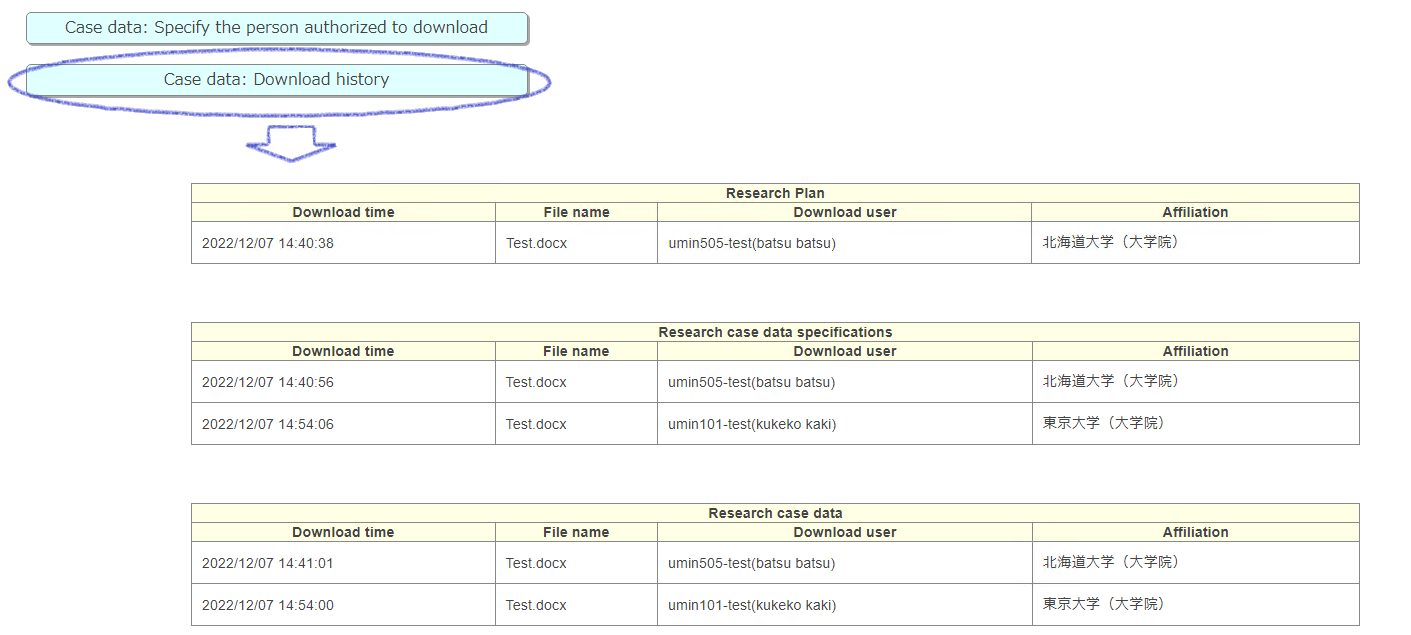
Case data: Download history
You can check the download date and downloader on the transition screen.
The content of this manual is the procedure for storing the data of "trials registered in UMIN-CTR" in the UMIN-ICDS system.
If you want to store only the data of trials registered in another clinical trial registration system, please refer to
"02.Archiving data from trials registered in other clinical trial registries".